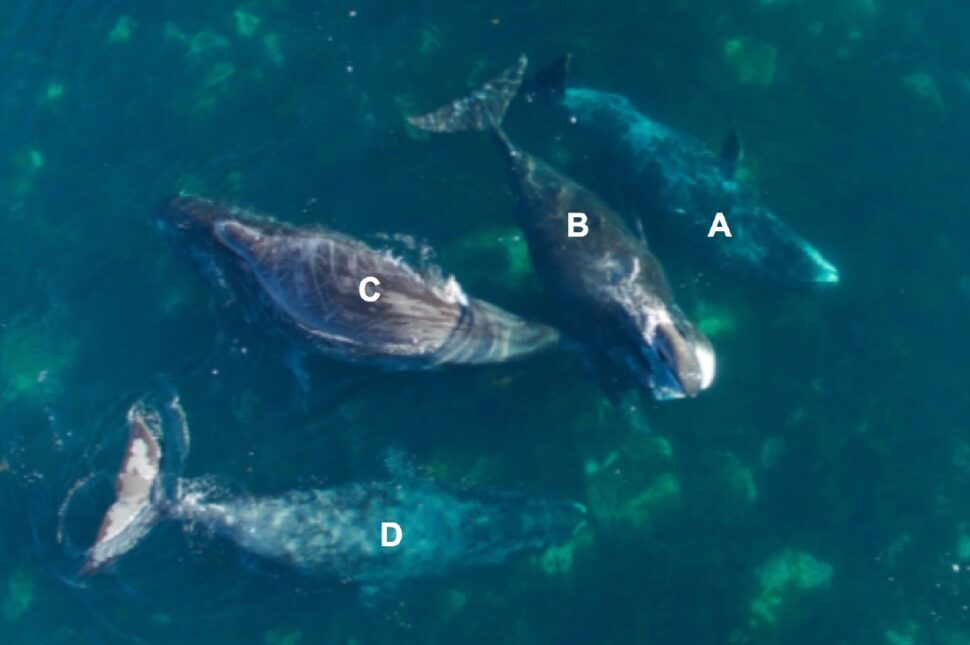
-

Oceanic Orcas: A new population of killer whales in the Northeast Pacific?
Evidence suggests these unknown orcas living in the open ocean may be unlike any other
0 -

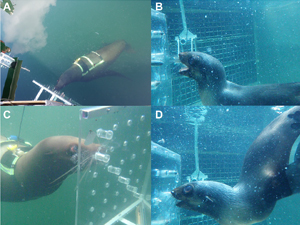
Aortic Bulbs: The secret to pinniped diving prowess?
Discover how this adaptation of the heart influences the diving abilities of seals and sea lions and what it means for their survival in a changing ocean
-
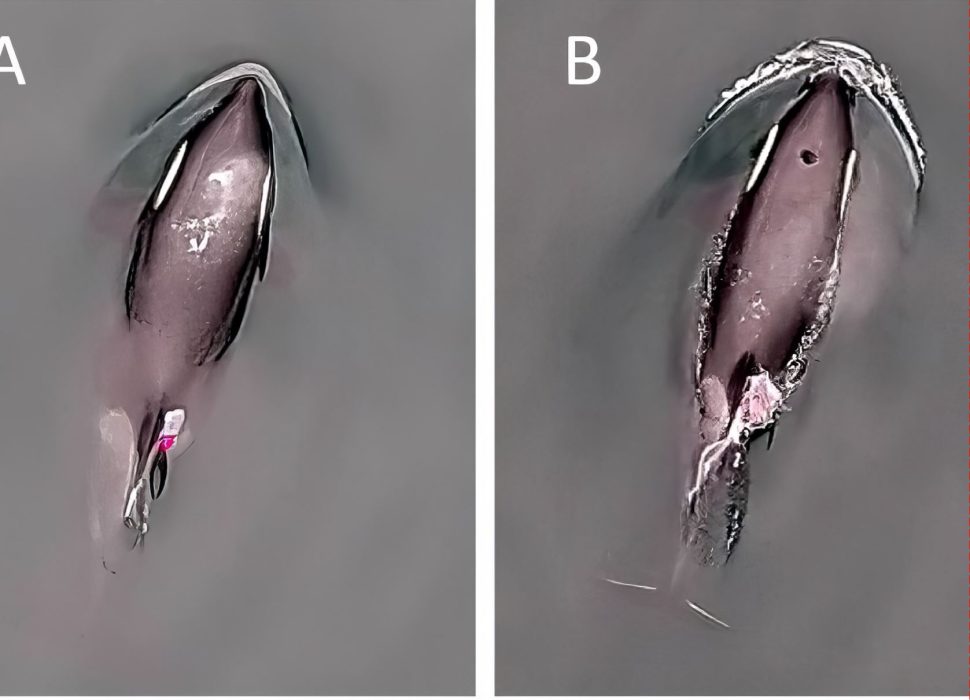
Breath by breath: What breathing rates tell us about the lives of killer whales
Killer whale respiration rates aren’t just about breathing—they’re a window into understanding their survival needs
-
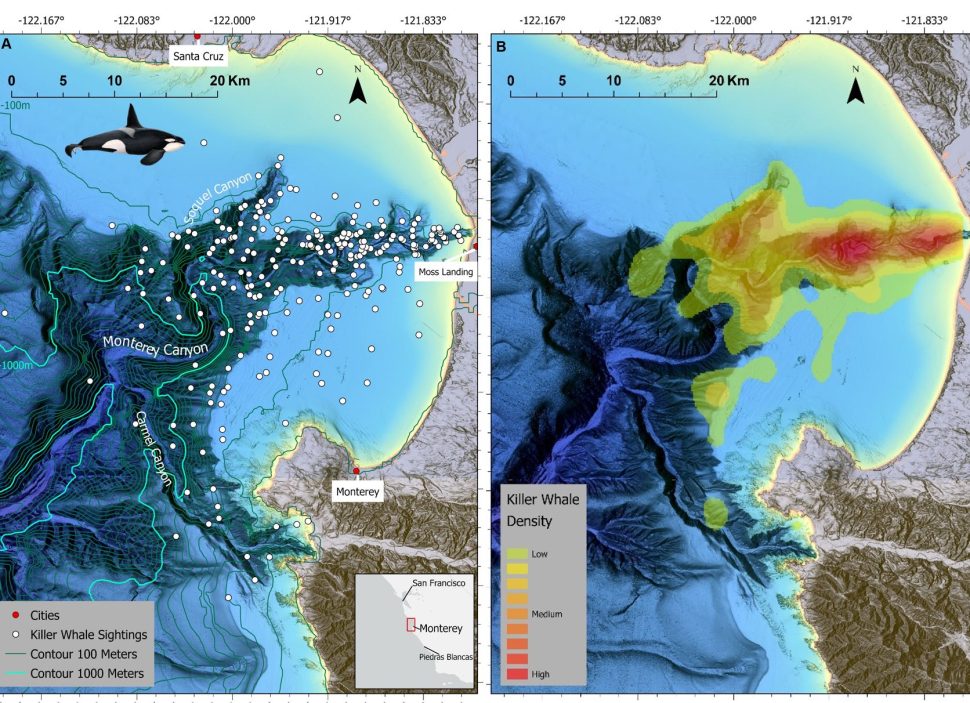
Foraging in the Abyss: Killer Whales and Submarine Canyons
Some killer whales have specialized in navigating submarine canyons to hunt marine mammals that exploit deep-sea species. How do they do it?
-
How do marine mammals make decisions about diving?
PhD candidate Rhea Storlund decided to take an unconventional approach to understand how marine mammals dive by asking human breath-hold divers about the decisions they make.
-
Grey Whales — the other Pacific Northwest resident whale
Grey whales face many threats ranging from entanglements and ship strikes, to loss of habitat and reduced prey availability. Researchers are collecting data this summer needed to quantify and mitigate these threats.
-

Northwest Student Chapter of the Society for Marine Mammalogy
Northwest Student Chapter of the Society for Marine Mammalogy Annual Conference was a Huge Success
-

It’s Been Two Years!
Last week graduate students from the Marine Mammal Research Unit attended their first in-person conferences in two years!
-

Decoding the secret lives of killer whales one micro-second at a time
Long gone are the days when a biologist could analyze a set of behavioural data by hand. Advancements in technology have put an end to that. Fortunately, teams of statisticians are coming to the rescue.
-

Grey Whale Watching in Baja California Sur, Mexico
It’s that time of year again, when people and grey whales head to the lagoons of Baja, Mexico for the annual winter event.
-

Long-term studies quantify the prey requirements of pinnipeds, and help predict the effects of nutritional stress.
Two new studies answer the question: “How much fish does a seal need?” One of the most common threats to marine mammal populations is reduced food supply, either due to the effects of climate change or human fishing pressure.
-

How big is that whale?
New data from stranded whales is yielding better estimates of body sizes needed to determine drug dosages, and assess the health and food requirements of whales.
-

You can’t beat a healthy heart
New research is shedding light on the hearts of healthy marine mammals, and how they compare to human hearts
-

No apparent shortage of prey for southern resident killer whales in the Salish Sea during summer
Researchers found four to six times more Chinook salmon in the Salish Sea in summertime compared with numbers of fish available to the growing population of northern resident killer whales.
-

2021 MMRU Students
After more than a year of working remotely, our graduate students are starting to head back to campus. They have been busy studying grey whales, killer whales, sea otters, sea lions, and much, much more….
-
What do differences in animal behaviour reveal about the decline of Steller sea lions in Alaska?
More than 50 years of studying Steller sea lion behaviour has yielded one of the most complete life history descriptions for any species of marine mammal.
-

The Killers of California and Oregon
Thirteen years of photo-identification data of killer whales observed in California and Oregon provide new insights into the distribution and population structure of mammal-eating killer whales in the eastern North Pacific Ocean.
-

Blue herons identified as a top juvenile salmon predator
It is more than just seals that are preying on the bounty of juvenile salmon exiting river mouths each spring.
-

How to Power A Walrus
New study shows loss of sea ice will require walruses to swim more and eat more to survive climate change
-

Harbour seals respond differently to pulses of out-migrating coho and Chinook salmon smolts
Biologging data from foraging harbour seals shows less impact on outmigrating salmon than expected. A few seals in the study…
-

SWIMMING WITH THE POD
For the past two weeks, UBC Researchers led by Dr. Andrew Trites have been studying the feeding behaviours of northern resident killer whales.
-

Bowhead whales feed year-round in Cumberland Sound, Nunavut
Satellite telemetry and time-depth recorders are providing new and surprising insights into the secret lives of bowhead whales Bowhead whales…
-

GO WALRUS GO!
Dr. David Rosen, an Assistant Professor at UBC’s Marine Mammal Research Unit, has often contended that walrus should be the…
-

A Window into the Lives of Resident Killer Whales
This summer, a team of researchers from the University of British Columbia, together with the Hakai Institute, set out to…
-

27th Annual BC Marine Mammal Symposium
Saturday November 23, 2019 27th AnnualB.C. MARINE MAMMAL SYMPOSIUMSaturday, November 23, 2019 – 9:30 am – 5:00 pm UNIVERSITY OF…
-

Struggle in the Strait
Is seal predation driving salmon declines in the Strait of Georgia? To many people, the sight of a harbor seal…
-

Do Foraging Strategies Matter?
Are female northern fur seals finding enough food to help their pups survive? The first few months at sea are…
-

-

-

26th Annual B.C. MARINE MAMMAL SYMPOSIUM
Saturday November 24, 2018 Join us for presentations as well as discussion on issues that concern us all. This meeting…
-

Drawing First Blood
Researchers publish first reference ranges for Steller sea lions Over the past 15 years, a small group of Steller sea…
-

13th LARKIN LECTURE
Climate contributions to hard times in US West Coast salmon fisheries SPEAKER: Nate Mantua NOAA NMFS Southwest Fisheries Science Center…
-

Dr. Andrew Trites wins the Timothy R. Parsons Medal
Dr. Andrew W. Trites (on left) – receives award from Andrew Stewart who represented DFO. Congratulations to our very own…
-

Into the Field
Bowhead rubbing Sometimes the coolest things happen, when you least expect it. These four bowhead whales…
-

This Just In
7 new publications… Molting bowhead whales, northern fur seal diets, killer whale foraging behavior, measuring stroke rates of sea lions…
-
Science Inreach
Oil painting “Hide and Seek” by Bruce Muir (2017) showing a southern resident killer whale pursuing Chinook salmon. Workshop held…
-

Science Outreach
SMM and AMSS Conferences A full house gathered in a hockey rink in eastern Canada as the pinniped scientists took…
-

From the Lab
How fat is that sea lion? Estimating the body condition of Steller sea lions may be as simple as pushing…
-

Fur Seals in Focus
Studying Acceleration and Energetics… in 3-D A fur seal “fitness tracker” called a Daily Diary Tag…
-

25th Annual B.C. MARINE MAMMAL SYMPOSIUM
Watch the Symposium from November 25, 2017: https://youtu.be/KtoEaTNkAfY Join us for presentations as well as discussion on issues that concern…
-

Into the Field: Harbor seals prey on salmon smolts
Hassen Allegue uses his laptop computer to program one of the biologging tags before deploying it…
-

-

August 2017 – This Just In
7 new publications… [webref_pretty title=”Accelerometers can measure total and activity-specific energy expenditure in free-ranging marine mammals only if linked to…
-

Science Outreach – August 2017
Science Outreach 21st Annual meeting of the Northwest Student Chapter of Marine Mammalogy Nearly 60 people from BC, Washington and…
-
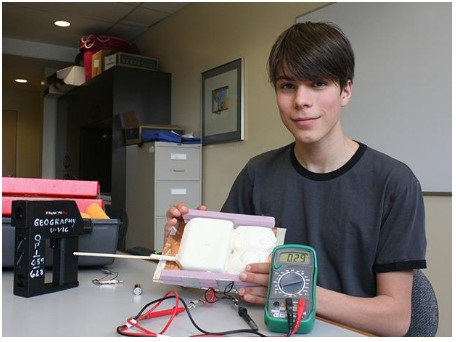
August 2017 – Off the Bench
Off the Bench Nattan Telmer shows some of the equipment he used to test the ability…
-

Steller Research Front and Center
Steller sea lion research at the Vancouver Aquarium is undergoing a major overhaul! The sea lions at the Aquarium moved…
-

-

Course Correction
Statistics brings fur seal foraging trips into focus For northern fur seals, a foraging trip in the Bering Sea can…
-

A Window to the Wild
Open Water Research Station Contributes a Decade of Discoveries This year marks the ten-year anniversary of the Open Water Research…
-

On-line Marine Mammal Symposium
The 24th Annual BC Marine Mammal Symposium was streamed on YouTube Live, Saturday, November 26 2016. About 230 people attended…
-

FROM THE LAB
Diving Hard at It Sitka, one of four Steller sea lions, has participated in many research…
-

24th Annual B.C. MARINE MAMMAL SYMPOSIUM
Saturday, November 26, 2016 – 9:30am – 5:00pm UNIVERSITY OF BRITISH COLUMBIA AQUATIC ECOSYSTEM RESEARCH LABORATORY GROUND FLOOR, AERL, 2202…
-

ROLLING IN THE DEEP
New Behaviors Observed in Northern Fur Seals Thanks to the emergence of new technologies in tagging, scientists are able to…
-

Analyzing Appetites in Harbor Seals
New DNA technique offers dietary insights Of all the species that share the North Pacific Ocean with humans, among the…
-

Dietary Dilemma
Which prey offer the greatest energetic return? In the remote reaches of the Bering Sea, the Pribilof Islands were once…
-

High-Tech Tracking in the Bering Sea
Electronic tags reveal secrets of foraging fur seals Northern fur seals in the eastern Bering Sea spend most of their…
-
Evolution of a Hunter
Do feeding strategies drive resilience? Many thousands of years ago, the ancient ancestors of today’s seals and sea lions moved…
-

Decisions at Depth: Does prey availability drive foraging behavior?
Sitka, one of 4 Steller sea lions trained to wear a harness carrying scientific instruments while…
-

12th LARKIN LECTURE: What the past tells us about the future of fisheries and oceans research in British Columbia
Dr. Richard Beamish From the Pacific Biological Station, Nanaimo, BC Thursday, October 29, 2015 2:00 – 4:00 pm Openhouse: Learn about IOF…
-

23rd Annual B.C. MARINE MAMMAL RESEARCH SYMPOSIUM
Saturday November 28, 2015 9:30am – 17:30pm Join us for presentations as well as discussion on issues that concern us…
-

The Science of Stress: A Nutrigenomic Approach
Feeling stressed? It’s a common complaint, but stress isn’t a uniquely human condition. Marine mammals also experience stress, especially when…
-

The Long View: A Historical Perspective on Steller Sea Lion Declines
image caption: An Aleut hunter in the Bering Sea with an anchored Russian ship in the background (watercolor by Louis…
-

Cool Waters, Warm Bodies
image caption: Two northern fur seal pups amongst sleeping adult females on the Pribilof Islands. Are young northern fur seals…
-

Is predation by harbor seals on juvenile fish responsible for the poor recovery of salmon?
Into the Field Chinook and coho are two of British Columbia’s most valuable salmon species. However, their numbers are at…
-

July 2014 – From the Lab to the Field
Counting salmon smolts as they pass down the throat of a seal one fish at a time Have you ever…
-

July 2014 – Science Outreach
Diving into beer and suds — marine mammal style! Beer and biology are well known associates on an individual level,…
-

July 2014 – This Just In
Steller sea lion diving awarded Gold Medal For more than 125 years, one gold medal has been awarded each year…
-

Running on Empty
Does nutritional status influence diving behavior in Steller sea lions? If you have ever searched for a gas station while…
-

Foraging in Fine-Scale
Pups and adult female northern fur seals look on as a bull makes threatening calls. New research details northern fur…
-

Calculating the limits of diving
Carling Gerlinsky on the diving platform with Hazy and two Steller sea lion trainers from the Vancouver Aquarium. The diving…
-

21st Annual B.C. MARINE MAMMAL SYMPOSIUM
Saturday, November 23, 2013 – 9:30am – 5:00pm UNIVERSITY OF BRITISH COLUMBIA AQUATIC ECOSYSTEM RESEARCH LABORATORY GROUND FLOOR, AERL, 2202 MAIN…
-

Predators in the Prey Patch
Northern fur seals at night New insights into the complex relationship between fur seals and pollock A multidisciplinary team of…
-

Measuring Diet in the Lab: The Pros and Cons of QFASA
At the heart of unraveling the precipitous decline of Western Alaska’s Steller sea lions are two key questions: What do they…
-

Eating right – key to survival of whales and dolphins
In the marine world, high-energy prey make for high-energy predators. And to survive, these marine predators need to sustain the…
-

Standing Out from the Crowd
How does scientific marking and tagging affect marine mammals? For decades, field researchers have applied paints, dyes, brands and telemetry…
-

Predator Puzzle: New evidence for predation as key factor in Steller Sea Lion collapse
Markus Horning spent the summer of 1998 in the remote Aleutian Islands, conducting research aboard the research ship M/V Tiglax.…
-

Meals for Seals: Maximum rates of fish intake in northern fur seals
Every October in Alaska’s remote Pribilof Islands, thousands of four-month-old northern fur seals take to the frigid waters of the…
-

What’s in a Whistle?
Transient killer whale with Steller sea lions on the rocks. New study examines how transient killer whales communicate first published March…
-

Genes in a Bottle
Using DNA analysis to solve a dietary dilemma The mysterious decline of Steller sea lions in Western Alaska presents a…
-

Metabolic Mysteries: Researchers explore connection between heart rate and energy expenditure
Scientists believe the precipitous decline of Western Alaska’s sea lion populations is driven by changes in the availability of their…
-

Diving for Dinner: The mechanics of foraging and commuting in Steller sea lions
As Steller sea lions decline in parts of the North Pacific Ocean, Consortium scientists are working to understand how sea…
-

Portrait of a Predator: Photo study leads to estimate of killer whale population
NE Pacific Transient killer whales in the Aleutian Islands, Alaska. Photograph taken during research conducted under permit 782-1719 issued by…
-

Go Go Gadget on the Go
The sea lions and fur seals in the Consortium’s captive research program work with researchers to test hypotheses that explain…
-

Northern fur seals – a Tale of Two Critters
A recent experience with young northern fur seals resulted in unexpected insights into the physiology of fur seals, and stronger…
-

Go Go Gadget Accelerometer
It fits in the palm of your hand, looks straight out of a James Bond film, and promises to help…
-

Endocrine Explorations
To land-dwellers, a sea lion’s life looks fairly uneventful: swim, eat, sleep and reproduce. In fact, a sea lion leads…
-

Laboratory Studies – One More Piece of the Puzzle
Scientific questions — like whether nutritional stress has led to the decline of Steller sea lion populations — are too…
-

Decisions, Decisions
As the far lesser-known adage goes, “It ain’t easy being marine.” This especially applies to the wild Steller sea lion,…
-

Scat Science
Researchers with their loot of scat It might not be the most glamorous aspect of a biologist’s work, but the…
-

Seasonal Differences in Biochemical Adaptation to Fasting in Juvenile and Subadult Steller Sea Lions
The Fasts of Life Unappealing as it may be to most humans, fasting is a fact of life for Steller…
-

Ecopath, Virtual Ecosystems, and the Status of the Gulf of Alaska
Ecopath was conceived by Dr. Polovina (NOAA) and advanced by Drs. Pauly, Christensen and Walters (UBC Fisheries Centre) — and…
-

Putting a Price Tag on Habitat Conservation
Protecting natural habitats rarely occurs without sparking controversies—usually among those whose livelihoods depend on resources within the protected areas. Conservation…
-

Summertime Blues
Does time of year affect recovery from nutritional stress? Winter in the North Pacific Ocean is considered the most difficult…
-

Reconstructing the Past: Prehistoric data helps to assess modern Pacific cod fishery
Pacific cod are among the most heavily fished species in the Northern Hemisphere, and once-thriving populations have significantly declined in…
-

Critical Habitat Revisited
Researchers Develop Predictive Model to Improve Legislation Federal laws that designate so-called Critical Habitat for Steller sea lions have played…
-

Deconstructing the Diet
New Model May Help to Quantify Prey Consumption Harbor seals play an important predatory role in the North Pacific Ocean,…
MARINE MAMMAL RESEARCH UNIT - UBC